Do you desperately look for 'combustion ethanol essay'? All the details can be found on this website.
Table of contents
- Combustion ethanol essay in 2021
- Ethanol combustion energy
- Complete combustion of ethanol
- Combustion of alcohols experiment conclusion
- Enthalpy of combustion chemistry ia
- Why is there a regular increase in enthalpies of combustion from methanol to ethanol to propan-1-ol
- Ethanol combustion formula
- Combustion of fuel experiment
Combustion ethanol essay in 2021
 This image shows combustion ethanol essay.
This image shows combustion ethanol essay.
Ethanol combustion energy
 This image representes Ethanol combustion energy.
This image representes Ethanol combustion energy.
Complete combustion of ethanol
 This image demonstrates Complete combustion of ethanol.
This image demonstrates Complete combustion of ethanol.
Combustion of alcohols experiment conclusion
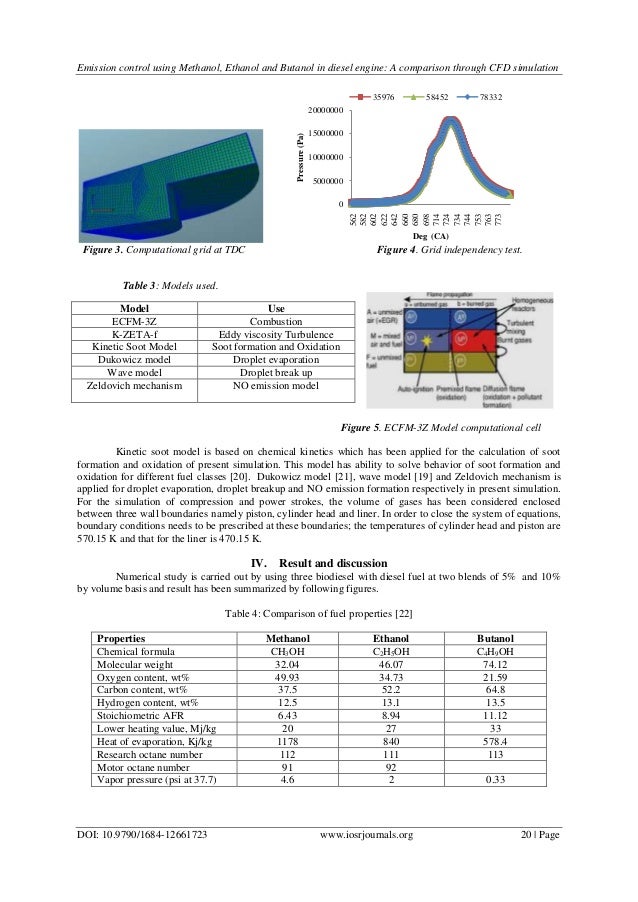 This image illustrates Combustion of alcohols experiment conclusion.
This image illustrates Combustion of alcohols experiment conclusion.
Enthalpy of combustion chemistry ia
 This image illustrates Enthalpy of combustion chemistry ia.
This image illustrates Enthalpy of combustion chemistry ia.
Why is there a regular increase in enthalpies of combustion from methanol to ethanol to propan-1-ol
 This picture representes Why is there a regular increase in enthalpies of combustion from methanol to ethanol to propan-1-ol.
This picture representes Why is there a regular increase in enthalpies of combustion from methanol to ethanol to propan-1-ol.
Ethanol combustion formula
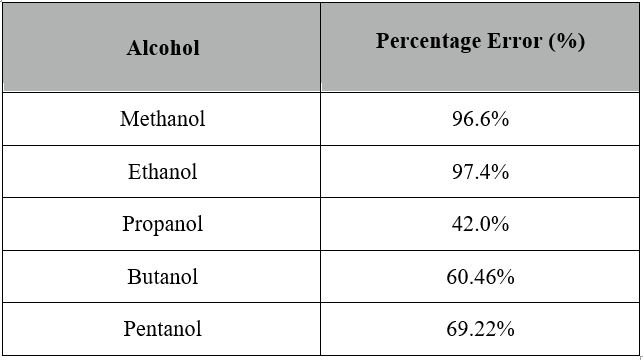 This image representes Ethanol combustion formula.
This image representes Ethanol combustion formula.
Combustion of fuel experiment
 This image representes Combustion of fuel experiment.
This image representes Combustion of fuel experiment.
Which is more efficient ethanol or methanol ignition temperature?
Through thorough experiments it would be more efficient to use ethanol because, the heat of combustion of ethanol is 1367 KJ/mole, ignition temperature of 363oC and flash point of 13 oC. Whereas Methanol’s heat of combustion is 724 KJ/mole, ignition temperature of 455oC and flash point of 12 oC. 10 pages, 4740 words
What is the heat of combustion of ethanol?
Whereas Methanol’s heat of combustion is 724 KJ/mole, ignition temperature of 455oC and flash point of 12 oC. Since ethanol has a higher heat of combustion you would need to use 3 times the amount of methanol to get the same amount of energy produced from ethanol.
Which is the best fuel for the combustion of alcohol?
The theoretical values emphasize the linear relationship between the enthalpy change and the increasing number of carbons in the alcohol. As a result it can be seen that methanol requires least energy to be burnt and therefore it acts as a best source of fuel out of the 5 alcohols investigated in this experiment.
Why are combustion of alcohols, lab report example?
This can be attributed to the weaknesses and limitations that are associated with this experiment. The major weakness that is associated with the experiment is human error. As a result of errors in the collection of data, there a significant disparity in the results obtained from the published results.
Last Update: Oct 2021