Are you having trouble finding 'comparison between cyclohexane and cyclohexene essay'? Here you can find your answers.
The key difference betwixt cyclohexane and cyclohexene is that the cyclohexane is A saturated hydrocarbon whereas the cyclohexene is an unsaturated hydrocarbon. There are different types of constitutional compounds made aside joining different elements with carbon atoms.
Table of contents
- Comparison between cyclohexane and cyclohexene essay in 2021
- Cyclohexene
- Cyclohexane ketone
- Cyclohexanone pka
- What is cyclohexanone used for
- Cyclohexane
- Cyclohexanone functional group
- Comparison between cyclohexane and cyclohexene essay 08
Comparison between cyclohexane and cyclohexene essay in 2021
 This image shows comparison between cyclohexane and cyclohexene essay.
This image shows comparison between cyclohexane and cyclohexene essay.
Cyclohexene
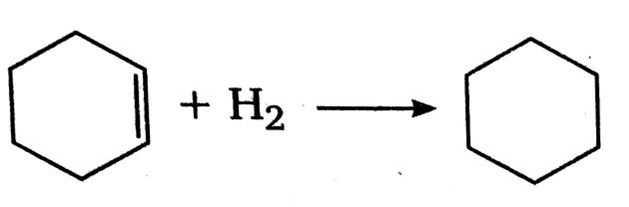 This image illustrates Cyclohexene.
This image illustrates Cyclohexene.
Cyclohexane ketone
 This image representes Cyclohexane ketone.
This image representes Cyclohexane ketone.
Cyclohexanone pka
 This picture representes Cyclohexanone pka.
This picture representes Cyclohexanone pka.
What is cyclohexanone used for
 This image representes What is cyclohexanone used for.
This image representes What is cyclohexanone used for.
Cyclohexane
 This picture illustrates Cyclohexane.
This picture illustrates Cyclohexane.
Cyclohexanone functional group
 This picture representes Cyclohexanone functional group.
This picture representes Cyclohexanone functional group.
Comparison between cyclohexane and cyclohexene essay 08
 This image representes Comparison between cyclohexane and cyclohexene essay 08.
This image representes Comparison between cyclohexane and cyclohexene essay 08.
What happens when Cyclohexene is exposed to light and air for a longer period?
When it is exposed to light and air for a longer period, it forms peroxides. We can produce this compound by hydrogenation of benzene until one double bond remains. Apart from that, it is a highly flammable liquid. Since cyclohexene has a double bond, it can undergo reactions that are characteristic of alkenes.
What is the difference between a cycloalkane and a cycloalkene?
Cycloalkanes are organic compounds having only single covalent bonds between carbon atoms in a ring structure; cyclohexane is a good example. Cycloalkenes, on the other hand, are organic compounds having singles bonds along with one or more double bonds between carbon atoms in the ring structure; cyclohexene is a good example.
What is the key difference between cyclohexane andcyclohexene?
The key difference between cyclohexane and cyclohexene is that the cyclohexane is a saturated hydrocarbon whereas the cyclohexene is an unsaturated hydrocarbon.
What is the chemical formula of cyclohexane and how is it produced?
Though it has the similar number of carbons like benzene, cyclohexane is a saturated hydrocarbon. So there are no double bonds or triple bonds between carbon atoms as in benzene. It is a colourless liquid with a mild, sweet odour. We can produce this compound by the reaction between benzene and hydrogen.
Last Update: Oct 2021