Are you hoping to find 'how to write redox reactions'? All material can be found on this website.
Table of contents
- How to write redox reactions in 2021
- Balancing redox reactions calculator
- Balancing redox reactions worksheet answers
- Redox practice worksheet
- 10 examples of redox reaction equations
- Redox reaction examples with answers
- Balancing redox reaction practice
- Redox reaction problems
How to write redox reactions in 2021
 This image demonstrates how to write redox reactions.
This image demonstrates how to write redox reactions.
Balancing redox reactions calculator
 This picture demonstrates Balancing redox reactions calculator.
This picture demonstrates Balancing redox reactions calculator.
Balancing redox reactions worksheet answers
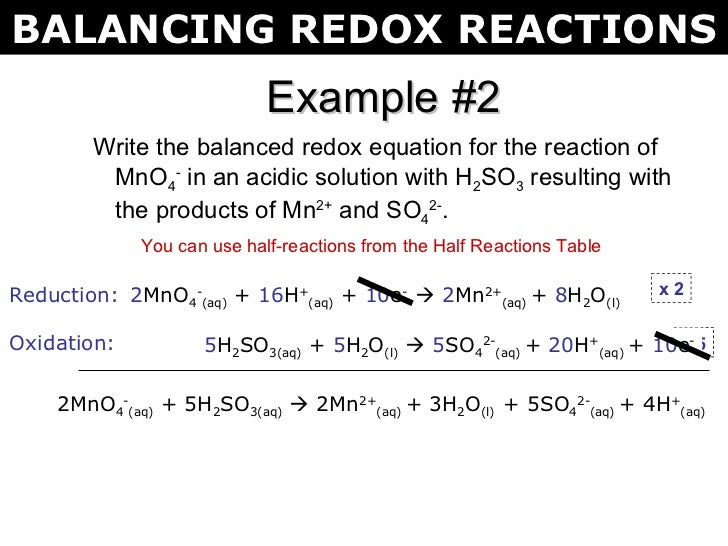 This picture shows Balancing redox reactions worksheet answers.
This picture shows Balancing redox reactions worksheet answers.
Redox practice worksheet
 This picture illustrates Redox practice worksheet.
This picture illustrates Redox practice worksheet.
10 examples of redox reaction equations
 This image representes 10 examples of redox reaction equations.
This image representes 10 examples of redox reaction equations.
Redox reaction examples with answers
 This image representes Redox reaction examples with answers.
This image representes Redox reaction examples with answers.
Balancing redox reaction practice
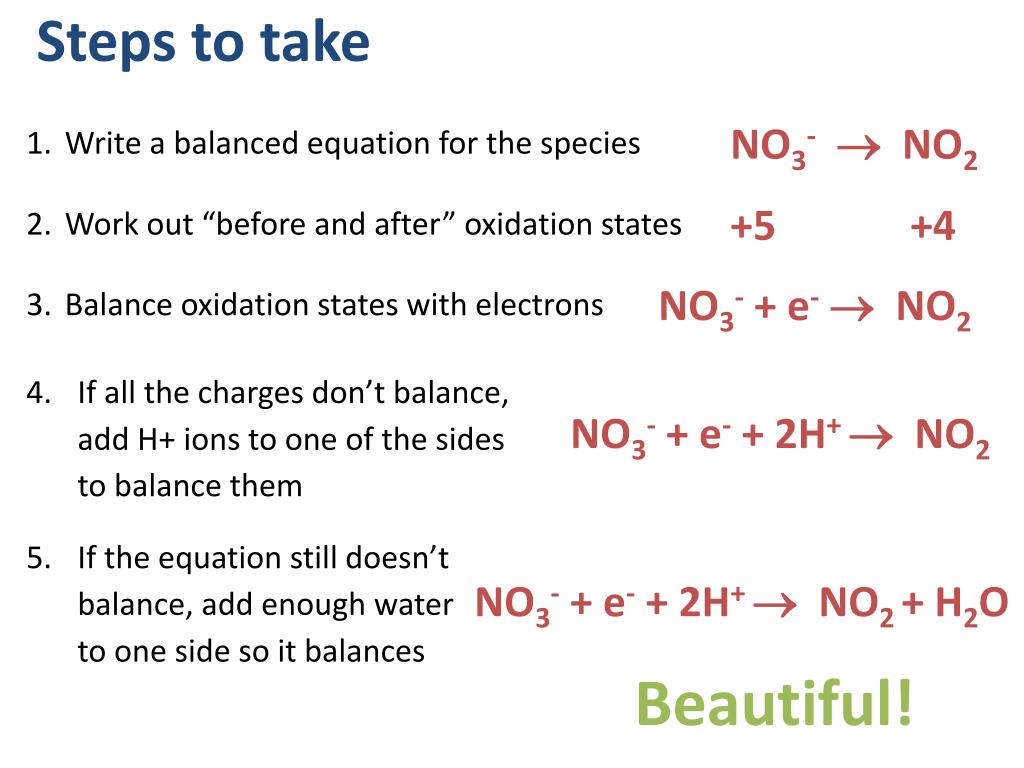 This picture shows Balancing redox reaction practice.
This picture shows Balancing redox reaction practice.
Redox reaction problems
 This image demonstrates Redox reaction problems.
This image demonstrates Redox reaction problems.
How are oxidation and reduction reactions broken down?
These species tend to undergo oxidation. It can be noted that any redox reaction can be broken down into two half-reactions, namely the oxidation half-reaction and the reduction half-reaction. When writing these half-reactions separately, each of them must be balanced in a way that all the electrons are accounted for.
What are the guidelines for balancing redox equations?
Guidelines for Balancing Redox Equations: Determine the oxidation states of each species. Write each half reaction and for each: Balance atoms that change oxidation state. Balance the number of electrons transferred for each half reaction using the appropriate factor so that the electrons cancel.
How are electrons gained and lost in a redox reaction?
Every redox reaction is made up of two half-reactions: in one, electrons are lost (an oxidation process); in the other, those electrons are gained (a reduction process). In the example above, the electron-half-equations were obtained by extracting them from the overall ionic equation.
How to write redox reactions and half reactions?
Galvanic and electrolytic cells 13.2 Writing redox and half-reactions (ESCQY) Redox reactions and half-reactions (ESCQZ) Remember from Grade 11 that oxidation and reduction occur simultaneously in a redoxreaction. The reactions taking place in electrochemical cells are redox reactions.
Last Update: Oct 2021
Leave a reply
Comments
Masaaki
20.10.2021 09:19Now, thanks to our popularity and clean image with users, our servers ar overwhelmed with clients' desperate pleas of write an essay for me patc our writing assessed homework redox reactions masterminds tend to their needs. T ste alance chares fashionable each half-reaction T ste 6 ealie electrons in all half-reaction t ste dd half-reactions toether.
Marygrace
21.10.2021 07:54Give in requirements to your assignment. Cheap paper penning service provides high-quality essays for low-cost prices.
Lorreen
28.10.2021 12:18The term reduction comes from the Italic stem meaning to lead back. Using the half-reaction method for solving redox equations.